Accelerating Time to Market for Software Driven Medical Devices. From concept to regulatory-compliant release.
Turning your medical device concept into a compliant, market-ready product. We navigate the regulatory maze of software development for medical devices so you can focus on innovation.
The Challenge
Great Tech doesn't always equal a Marketable, Compliant device.
You have a vision, or perhaps a prototype that works perfectly in the lab and small trials. But to get it to patients, you need to navigate IEC 62304, FDA guidance, and safety classifications. Where large consultancies offer expensive, rigid templates, Aritia offers flexible, experience-led strategy designed specifically for start-ups and SMEs.
Why Us
Worked with 150+ Teams: Experience across the full spectrum of devices, from diagnostics to safety-critical therapy systems.
Pragmatic, Not Dogmatic: We adapt to your size. No unnecessary paperwork, just what is required for safety and compliance.
Cost-Effective Expertise: High-level strategic advice without the overheads of a "Big 4" consultancy.

Services
Software Regulatory Strategy:
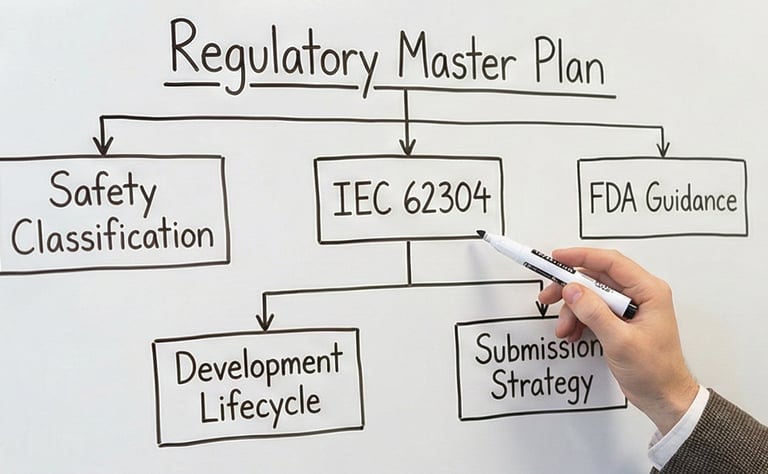
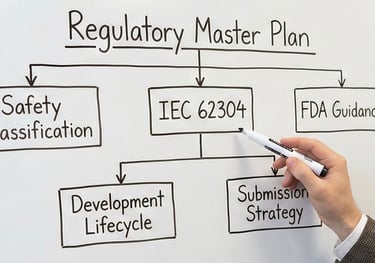
Establishing safety classifications and creating the "Master Plan" for your software development lifecycle.
Gap Analysis & Rescue:
Requirements & Risk Management:
Vendor Management & Oversight:
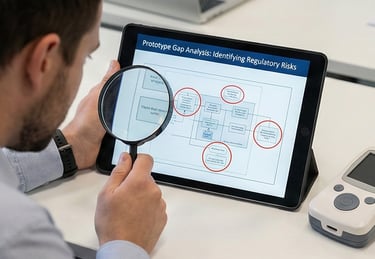
Reviewing existing prototypes or unstructured codebases to identify exactly what is missing for regulatory submission.
Acting as your "Technical Voice" to manage external software agencies, ensuring they deliver quality, compliant code.
Helping you define software requirements and risk controls (ISO 14971) before development begins (or retrofitting them if it has already started).